Schramm GAK, Waldmann-Rex S Effects of 2 mg Chlormadinone Acetate/0.03 mg Ethinylestradiol in Primary Dysmenorrhoea: The BEDY (Belara(R) Evaluation on Dysmenorrhea) Study - an Open, Non-Comparative, Non-Interventional Observational Study with 4,842 Women Journal für Reproduktionsmedizin und Endokrinologie - Journal of Reproductive Medicine and Endocrinology 2010; 7 (Sonderheft 1): 112-118 Volltext (PDF) Summary Übersicht
| ||||||||||||
Abbildung 6: BEDY Study - Cycle-Related Disorders Incidence of moderate to severe symptoms in other cycle-related disorders, before and after the administration of 2 mg chlormadinone acetate/0.03 mg ethinylestradiol (CMA/EE). Results from patients with valid ratings at baseline and after 6 cycles/blister packs of CMA/EE treatment are shown. |
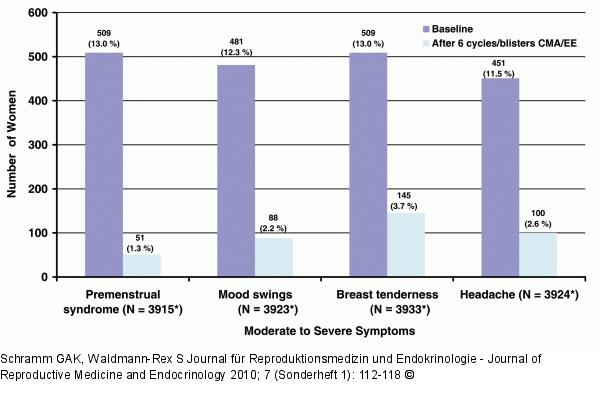
Abbildung 6: BEDY Study - Cycle-Related Disorders
Incidence of moderate to severe symptoms in other cycle-related disorders, before and after the administration of 2 mg chlormadinone acetate/0.03 mg ethinylestradiol (CMA/EE). Results from patients with valid ratings at baseline and after 6 cycles/blister packs of CMA/EE treatment are shown. |






