Waters J, Ashford J, Jäger B, Verboom CN, Wonnacott S Use of moxonidine as initial therapy and in combination in the treatment of essential hypertension - results of the TOPIC (Trial Of Physiotens In Combination) Study Journal of Clinical and Basic Cardiology 1999; 2 (2): 219-224 PDF Summary Overview
| ||
Figure/Graphic 1: TOPIC-Studie - Design Study plan for TOPIC. A = placebo run-in phase (4 weeks; n = 792). B = open-label monotherapy phase (8 weeks; n = 678; moxonidine 200 mcg/day for 4 weeks, then 200 or 400 mcg/day as required). C = moxonidine monotherapy (4 weeks; n = 303; moxonidine 200 or 400 mcg/day as required for 4 weeks). D, E, F = combination therapy (4 weeks; moxonidine [400 mcg/day] plus other agent as specified below). D = moxonidine plus amlodipine (5 mg/day) (n = 87). E = moxonidine plus enalapril (10 mg/day) (n = 88). F = moxonidine plus hydrochlorothiazide (12.5 mg/day) (n = 97). |
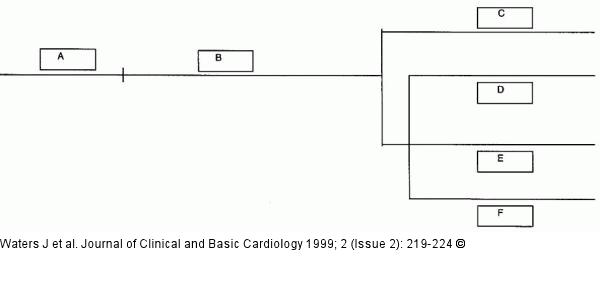
Figure/Graphic 1: TOPIC-Studie - Design
Study plan for TOPIC. A = placebo run-in phase (4 weeks; n = 792). B = open-label monotherapy phase (8 weeks; n = 678; moxonidine 200 mcg/day for 4 weeks, then 200 or 400 mcg/day as required). C = moxonidine monotherapy (4 weeks; n = 303; moxonidine 200 or 400 mcg/day as required for 4 weeks). D, E, F = combination therapy (4 weeks; moxonidine [400 mcg/day] plus other agent as specified below). D = moxonidine plus amlodipine (5 mg/day) (n = 87). E = moxonidine plus enalapril (10 mg/day) (n = 88). F = moxonidine plus hydrochlorothiazide (12.5 mg/day) (n = 97). |